Central Study Planning & Project Management
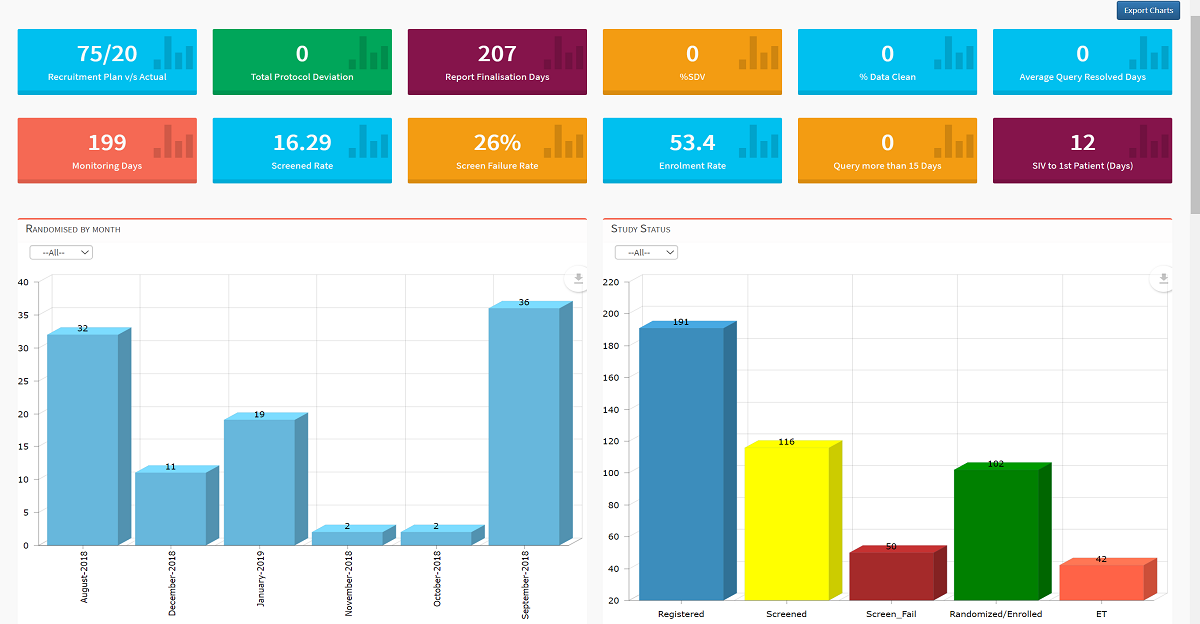
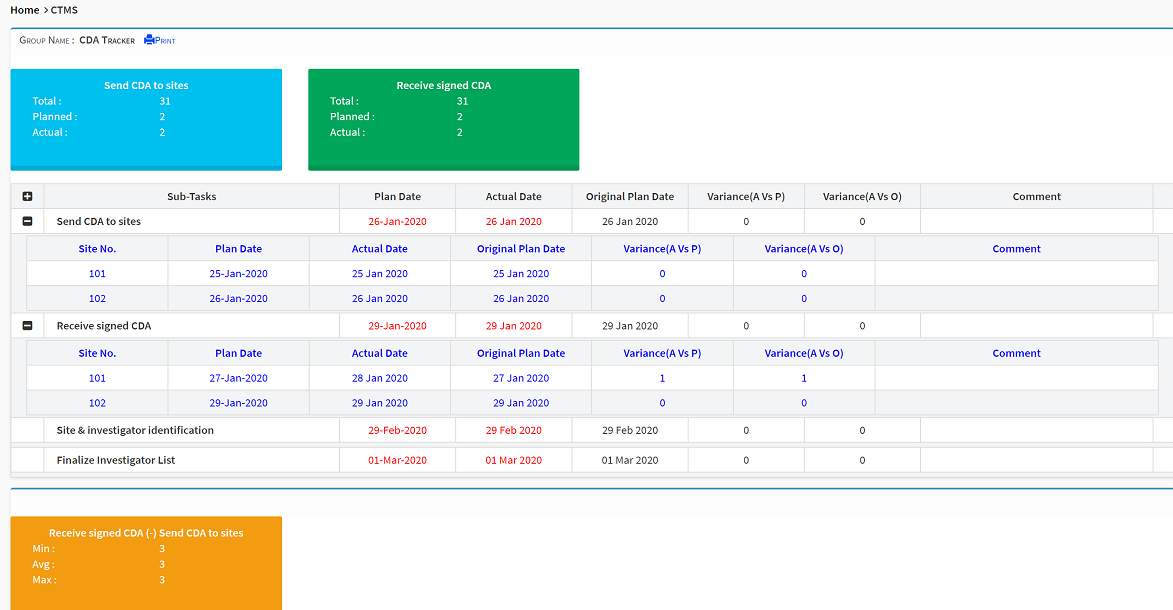
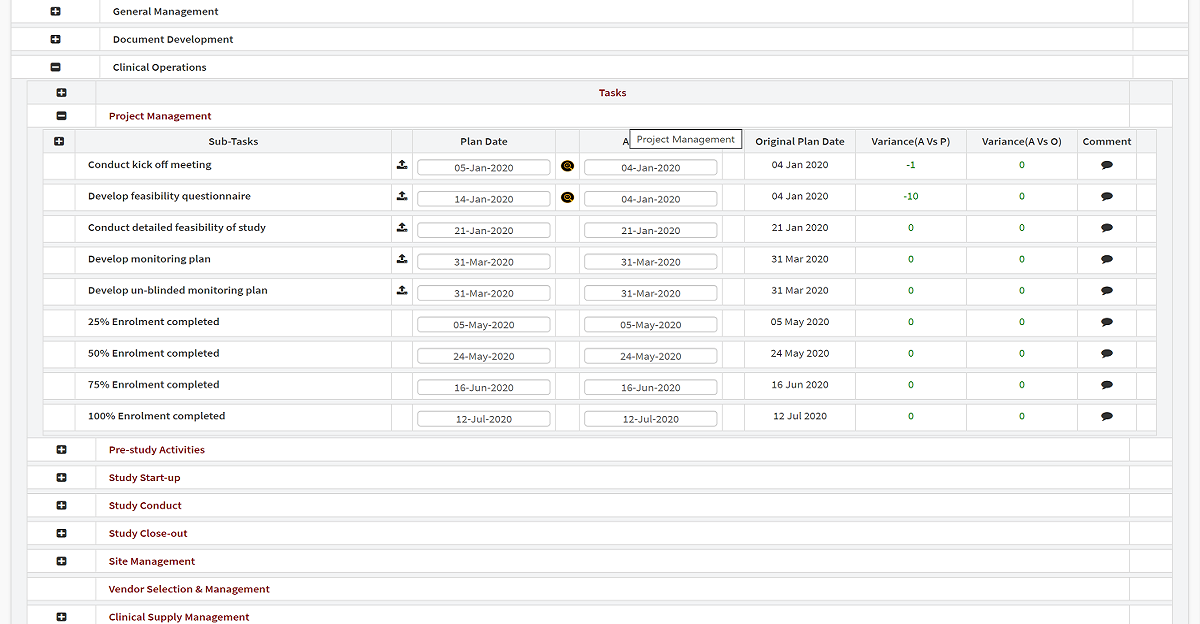
Completely user-configurable with dropdown menus, sans consultants, our end-to-end clinical trial management system delivers industry-leading comprehensive project management capabilities from inception to close. Active advanced analytics and custom workflows enable complete control over each clinical trial.