During development of new medicines, a lot of research data is generated, reviewed, analyzed and reported. The data is captured, generated, and tracked using various, usually disparate systems. These systems seldom have a handshake with each other and are not integrated. The data review is performed by various diverse teams like Medical personnel, data management teams, biostatisticians, clinical operations teams from time to time. This review of data is not fully coordinated between teams nor it is real-time and hence it contributes to late identification of risks to data quality, data integrity and to above all participating patients.
This gets complicated further as the number of trials and complexity has increased over time. Increased variability of clinical investigator experience, site infrastructure, challenges related to geographic dispersion and variable standards of care.
Ensuring Basic Principles of Clinical Research are Upheld All the Time Using Integrated Technology: Patient Safety, Patient Rights, Data Integrity, Data Quality and Transparency of Conduct
Conventional Approach
Conventional approach to risk management and patient safety is often fraught with review delays which can lead to reduced regulatory compliance. In last few years, drug regulators across the world have encouraged use of technological advances in data sciences to be used in pharmaceutical drug development, in order to identify risks and provide mitigation strategies in real-time. There, however, is still a large gap fully integrating all possible data sources and then to use medical, statistical, and operational algorithms to identify risks to patients, data quality and integrity.
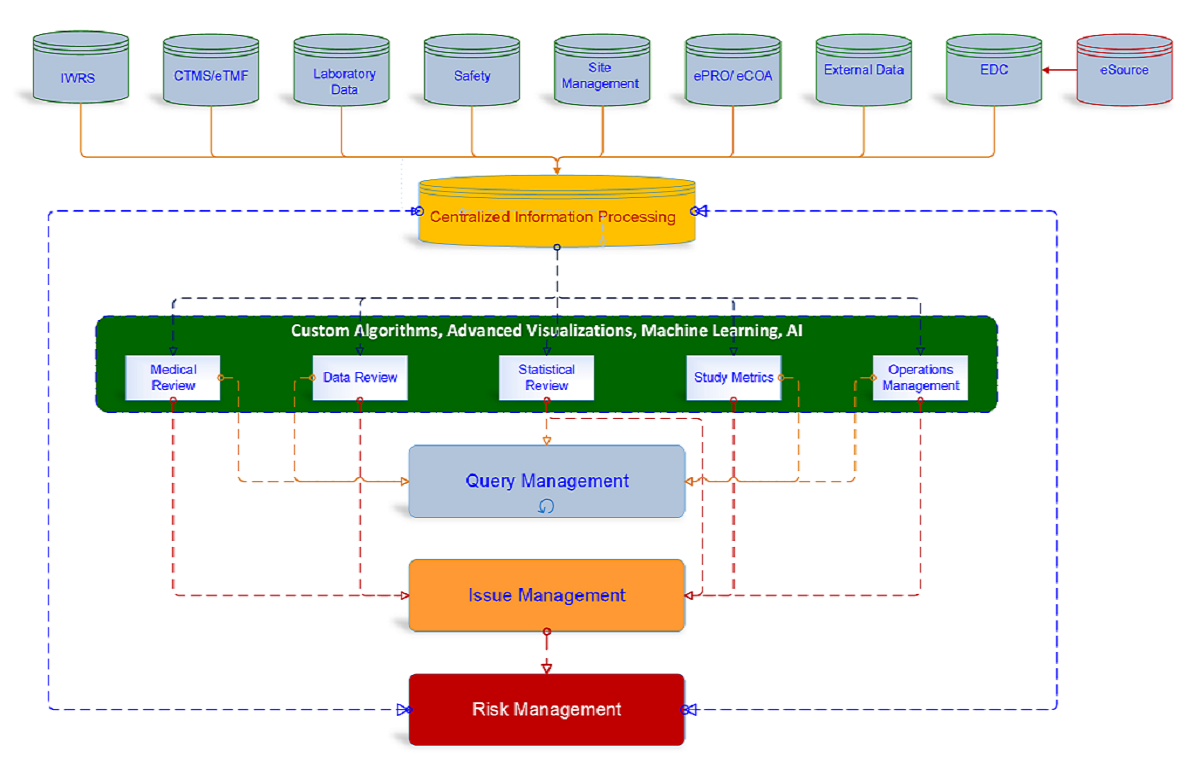
Wide-Angle-Insights - Next Generation Platform focused on Risk
Wide-Angle-Insights is a fully integrated, source agnostic, data sciences platform with intuitive customized algorithms, smart data reduction, advanced analytics & visualization to facilitate real-time 'signal detection, adjudication, correlation & management' and comprehensive issue management & risk management based on industry standards like TransCelerate as well as ISO 31010, while ensuring collaborative review efficiency and transparency.
Having been conceptualized and developed by actual users having significant clinical research experience across a broad spectrum of therapeutic areas, the Wide-Angle-Insights platform successfully addresses the shortcomings of standalone, generic clinical research management tools that operate in silos and even goes beyond leading unified platforms. The platform ensures significant reduction in data errors, early identification of risks and shorten development timelines (including early go/ no-go decisions). Operational & predictive analytics and machine learning gives users full control over clinical trial data. This enables users to achieve over 75% efficiency in resources and timelines.
In addition to conventional audit trails, Wide-Angle-Insights incorporates complete and printable duly classified event histories, a first in the industry. Moreover, the platform can also integrates emails, facilitate meetings and document decision making process. Automation and customized algorithms, coupled with a high degree of configurability, lead to enhanced efficiency and significant savings in terms of effort and cost.
