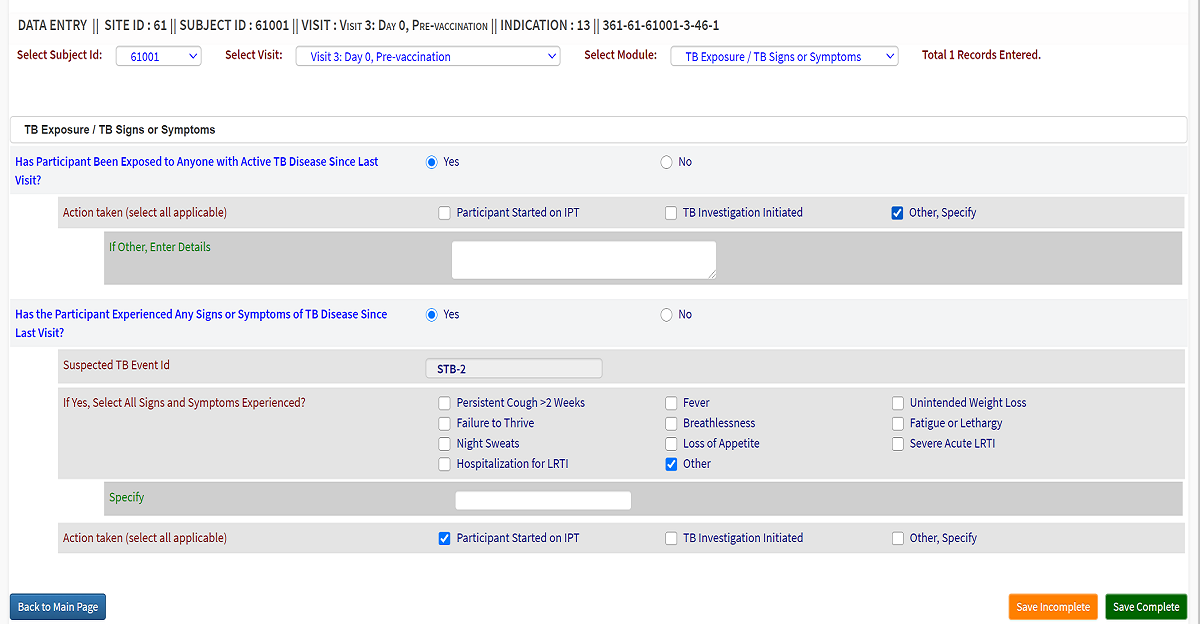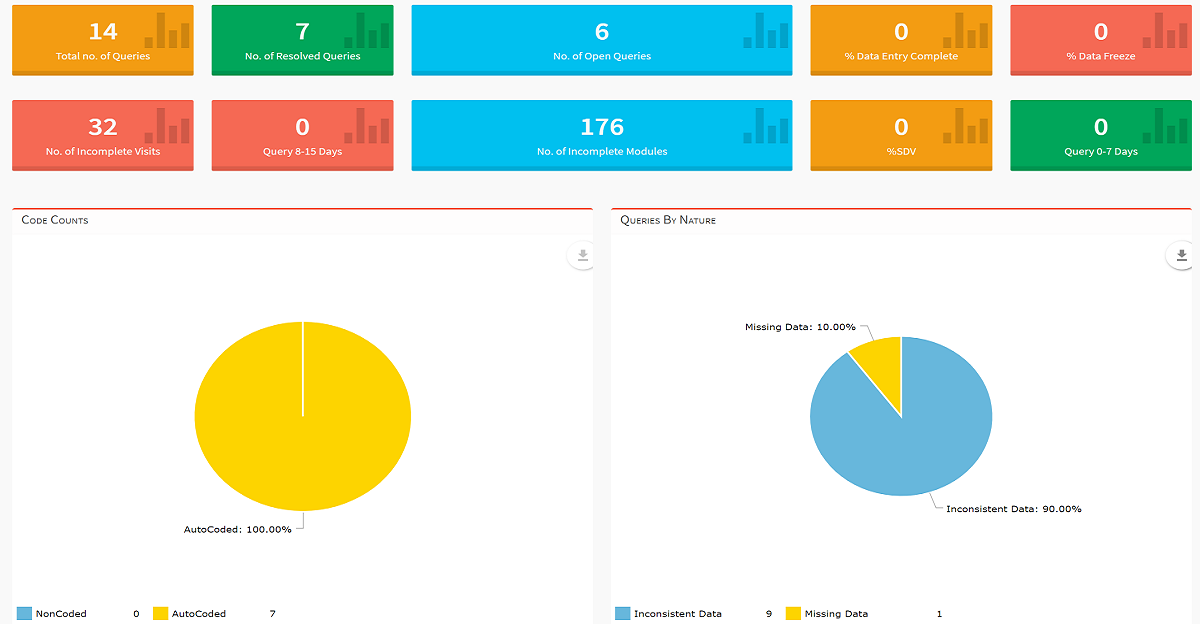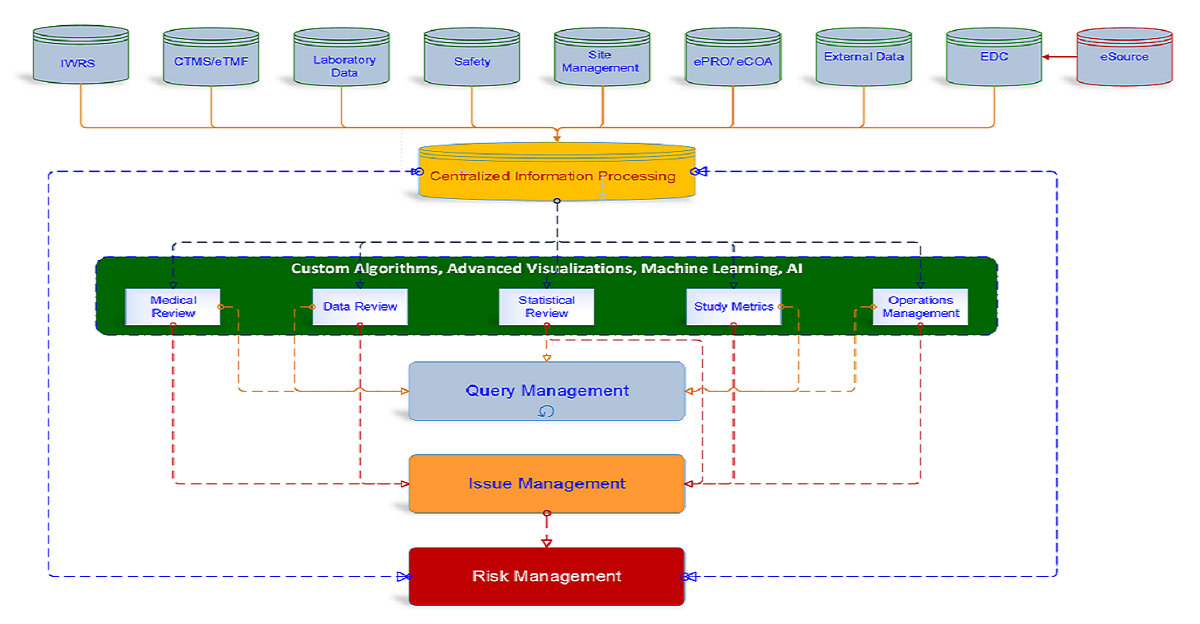
Intelligent Data Architecture
Build your eDC platform in less than 2 weeks. Intuitive database design, library of variables & drag and drop controls keeps eDC database structure lean and in compliance with Industry standards. Data Structure enables users to one click data export to analysis platform reducing programming effort on analysis platform saving valuable time and effort.